For Healthcare Professionals
FDA Registration and Clinical Trials
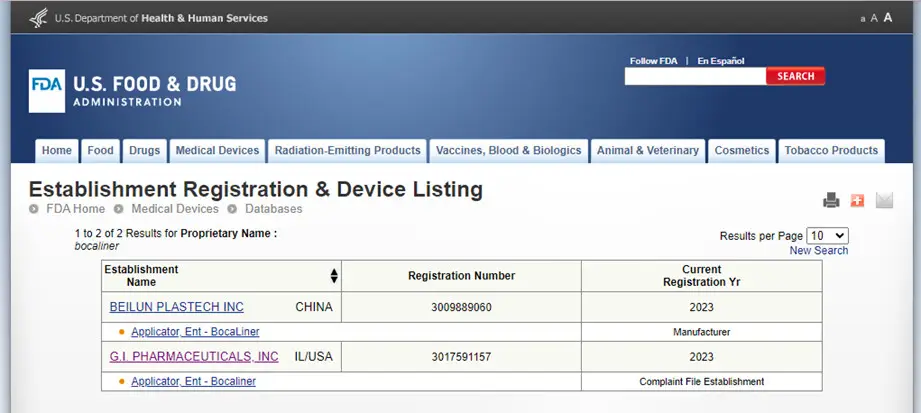
Bocaliner is registered with the Food and Drug
Administration as a Class 1 medical device
Clinical Trials
The Phase 1/Phase 2 Study for the Prevention of Oral Mucositis (SPOM) is an ongoing clinical trial. The trial has two parts. Both parts of the study are being conducted by the Oncology group at the Hematology Center after Professor R. Yeolyan in Yerevan, Armenia. The patients in the study are all undergoing systemic chemotherapy treatment for hematologic malignancies.
The first part of the study (Phase 1) is designed to evaluate the safety and tolerability of Bocaliner in these patients. Near completion, this study has shown that Bocaliner is safe and well tolerated in this group of patients, even after developing painful mouth sores from oral mucositis. Oral mucositis is a common side effect of cancer treatments, such as chemotherapy and radiation therapy, characterized by inflammation and ulceration of the oral mucosa.

The second part of the study is designed to investigate the use of benzydamine, a mouthwash with antimicrobial and anti-inflammatory properties to prevent oral mucositis in these patients. A group of patients in the study will also use Bocaliner together with benzydamine or saline mouthwashes to study how the Bocaliner can enhance the effect of these treatments in prevention of oral mucositis.
There are limited numbers of medical options available to prevent oral mucositis in patients that are undergoing chemotherapy. This clinical trial, registered under the identifier NCT05338398 on
www.clinicaltrials.gov is specifically designed to assess the ability of Bocaliner for helping to prevent oral mucositis in adult patients undergoing cancer treatment diagnosed with oral mucositis.
www.clinicaltrials.gov is specifically designed to assess the ability of Bocaliner for helping to prevent oral mucositis in adult patients undergoing cancer treatment diagnosed with oral mucositis.

